FOR IMMEDIATE RELEASE
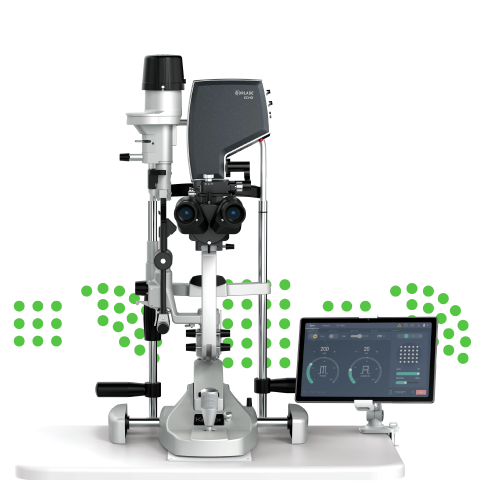
Ballerup, Denmark and Redwood City, CA, USA – October 28, 2020 – Norlase today announced the 510(k) clearance from the U.S. Food and Drug Administration and the immediate commercial launch of Norlase® LION™, a green laser photocoagulator fully-integrated into a Keeler indirect ophthalmoscope. Unlike other laser indirect ophthalmoscopes that require a fiber optic connection to a laser source, LION has no fiber tether, is powered by a battery, and utilizes an advanced wireless interface with voice control of parameters. With the new LION, ophthalmologists can experience an untethered, lightweight, and portable laser treatment solution in practically any setting.
“An FDA clearance and the successful launch of LION are not only gratifying but also validating as we continue to introduce lasers that improve practice efficiency and, most importantly, sets a new standard in eyecare,” said Oliver Hvidt, CEO and President of Norlase. “The LION green laser represents our deep commitment to create the highest value for ophthalmologists and the best care for patients. I am extremely proud of our team and excited as we move forward into 2021 with innovative products that shape the landscape of the eyecare industry.”
Product features include:
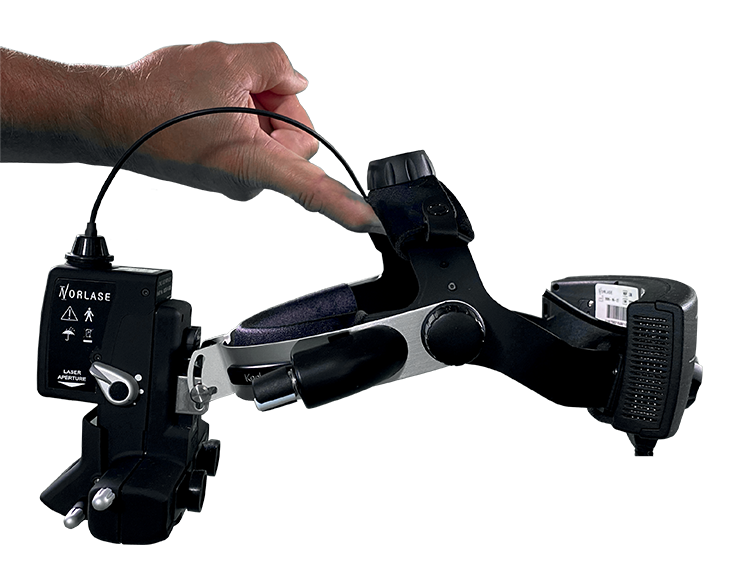 Green laser and LIO in one integrated device eliminating the need for an external laser console.
Green laser and LIO in one integrated device eliminating the need for an external laser console.- Untethered design with no fiber tether, reducing risk of fiber breakage and costly repair experienced by competitive products.
- Compact and portable design provides easy transport between treatment rooms or offices.
- Rechargeable battery provides more than a full day of continuous use.
- Parameter settings operable through an intuitive user interface on a wireless tablet.
- Optional voice recognition, providing touchless parameter control during treatment.
“The LION is truly revolutionary,” comments Dr. John Kitchens, Retina Associates of Kentucky. “Being able to do laser so efficiently in any location in a matter of moments has already fundamentally changed our clinic flow. Within a minute or two, I can now laser anyone, safely and reliably, anywhere.” Dr. S.K. Steven Houston III, Florida Retina Institute adds: “LION’s superior optics and enhanced visualization has greatly improved the efficiency of my practice. Combined with the portability, I am able to perform retinal laser treatments much faster – which my patients appreciate. This will be a game changer for my practice now and well into the future.”
LION is available and currently being sold in the United States. The company looks forward to expanding adoption of LION and its suite of smart laser solutions in the global retina market.
“LION is the culmination of advanced laser technology and thoughtful design based around optimizing the user experience and improving treatment efficiency,” says Greg Fava, Vice President, Medical Devices of Norlase. “It has been decades since the last major advancement in laser indirect ophthalmoscope design and LION has set the new standard. We will continue to work closely with ophthalmologists to unveil additional ophthalmic solutions with premiere features at an unmatched price.”
About Norlase
Norlase develops next-generation laser solutions for the treatment of retina and glaucoma diseases. Founded in Denmark, Norlase is comprised of worldwide industry experts in ophthalmology, laser technology, medical device development, and customer care. Norlase is on a mission to improve practice efficiency, patient care and physician convenience for ophthalmologists worldwide. Norlase entered the United States ophthalmic market with its flagship product LEAF, a laser photocoagulator that’s 10X smaller than existing systems and mounts directly on the slit lamp.
FOR IMMEDIATE RELEASE
Norlase Announces FDA Clearance and U.S. Launch of New LION Laser System
Ballerup, Denmark and Redwood City, CA, USA – October 28, 2020 – Norlase today announced the 510(k) clearance from the U.S. Food and Drug Administration and the immediate commercial launch of Norlase® LION™, a green laser photocoagulator fully-integrated into a Keeler indirect ophthalmoscope. Unlike other laser indirect ophthalmoscopes that require a fiber optic connection to a laser source, LION has no fiber tether, is powered by a battery, and utilizes an advanced wireless interface with voice control of parameters. With the new LION, ophthalmologists can experience an untethered, lightweight, and portable laser treatment solution in practically any setting.
“An FDA clearance and the successful launch of LION are not only gratifying but also validating as we continue to introduce lasers that improve practice efficiency and, most importantly, sets a new standard in eyecare,” said Oliver Hvidt, CEO and President of Norlase. “The LION green laser represents our deep commitment to create the highest value for ophthalmologists and the best care for patients. I am extremely proud of our team and excited as we move forward into 2021 with innovative products that shape the landscape of the eyecare industry.”
Product features include:
- Green laser and LIO in one integrated device eliminating the need for an external laser console.
- Untethered design with no fiber tether, reducing risk of fiber breakage and costly repair experienced by competitive products.
- Compact and portable design provides easy transport between treatment rooms or offices.
- Rechargeable battery provides more than a full day of continuous use.
- Parameter settings operable through an intuitive user interface on a wireless tablet.
- Optional voice recognition, providing touchless parameter control during treatment.

“The LION is truly revolutionary,” comments Dr. John Kitchens, Retina Associates of Kentucky. “Being able to do laser so efficiently in any location in a matter of moments has already fundamentally changed our clinic flow. Within a minute or two, I can now laser anyone, safely and reliably, anywhere.” Dr. S.K. Steven Houston III, Florida Retina Institute adds: “LION’s superior optics and enhanced visualization has greatly improved the efficiency of my practice. Combined with the portability, I am able to perform retinal laser treatments much faster – which my patients appreciate. This will be a game changer for my practice now and well into the future.”
LION is available and currently being sold in the United States. The company looks forward to expanding adoption of LION and its suite of smart laser solutions in the global retina market.
“LION is the culmination of advanced laser technology and thoughtful design based around optimizing the user experience and improving treatment efficiency,” says Greg Fava, Vice President, Medical Devices of Norlase. “It has been decades since the last major advancement in laser indirect ophthalmoscope design and LION has set the new standard. We will continue to work closely with ophthalmologists to unveil additional ophthalmic solutions with premiere features at an unmatched price.”
About Norlase
Norlase develops next-generation laser solutions for the treatment of retina and glaucoma diseases. Founded in Denmark, Norlase is comprised of worldwide industry experts in ophthalmology, laser technology, medical device development, and customer care. Norlase is on a mission to improve practice efficiency, patient care and physician convenience for ophthalmologists worldwide. Norlase entered the United States ophthalmic market with its flagship product LEAF, a laser photocoagulator that’s 10X smaller than existing systems and mounts directly on the slit lamp.

DIDN’T FIND WHAT YOU’RE LOOKING FOR?
Let us know how we can help!
DIDN’T FIND WHAT YOU
WERE LOOKING FOR?
Let us know how we can help!