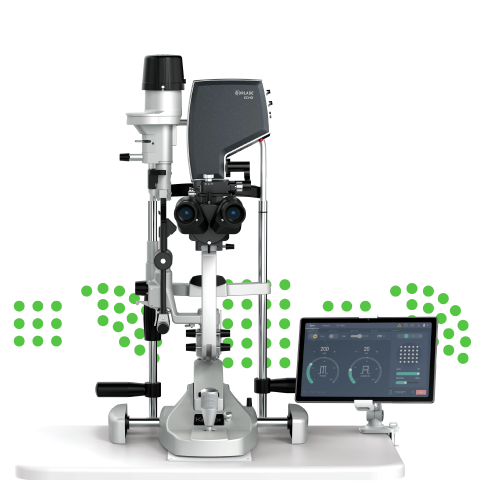
San Carlos, CA, USA – October 12, 2019 – Norlase today announced the FDA 510(k) regulatory clearance and immediate commercial launch of LEAF*, the company’s flagship laser product for the treatment of retina and glaucoma disease. LEAF allows ophthalmologists to perform laser therapy in almost any exam room, with minimal set-up time and physical space required. The entire laser unit attaches conveniently to an existing slit lamp, eliminating the need for a cart or counter space to house the laser console. Additionally, the device’s fiberless design reduces costly service repairs common with other laser technology.
Designed to give ophthalmologists unprecedented portability, reliability and efficiency, LEAF utilizes a wireless tablet as the user interface. An optional voice control feature, powered by Sensory Inc, provides touchless parameter control during treatment, allowing the physician to maintain focus on the patient.
Product features include:
- Ultra-compact and portable green laser
- No external fiber to damage or limit the practice space where it can be used
- Intuitive, sleek tablet user interface with wireless connectivity for maximum versatility
- Industry-first voice control for changing parameters
“Norlase is proud to launch LEAF to ophthalmologists looking for innovative technology,” said Greg Fava, Vice President, Medical Devices of Norlase. “In over twenty-five years working with laser photocoagulator technology, never has a product brought together the latest advancements in laser technology, wireless connectivity and voice control convenience. LEAF offers these product attributes at an unmatched price point. Additionally, Norlase plans on working closely with ophthalmologists to provide a comprehensive suite of laser solutions in the years to come.”
“The Norlase laser is so different from other photocoagulators you really have to see it to appreciate the advancements. It is extremely small and has no external fiber to break – eliminating a major repair expense common with other technologies,” comments John Kitchens, MD Retina Associates of Kentucky. The device’s remarkable portability makes it easy to integrate in practically any office environment.
About Norlase
Norlase develops next-generation laser solutions for the treatment of retina and glaucoma disease. Founded in Denmark, Norlase is comprised of worldwide industry experts in ophthalmology, laser technology, medical device development and customer care. Norlase is on a mission to improve practice efficiency, patient care and physician convenience for ophthalmologists worldwide. Norlase entered the United States ophthalmic market with LEAF, a new laser photocoagulator that’s 10X smaller than existing systems and mounts directly on the slit-lamp.
For more information, please visit www.norlase.com
*Norlase LEAF is available for distribution in the US.

DIDN’T FIND WHAT YOU’RE LOOKING FOR?
Let us know how we can help!
DIDN’T FIND WHAT YOU
WERE LOOKING FOR?
Let us know how we can help!